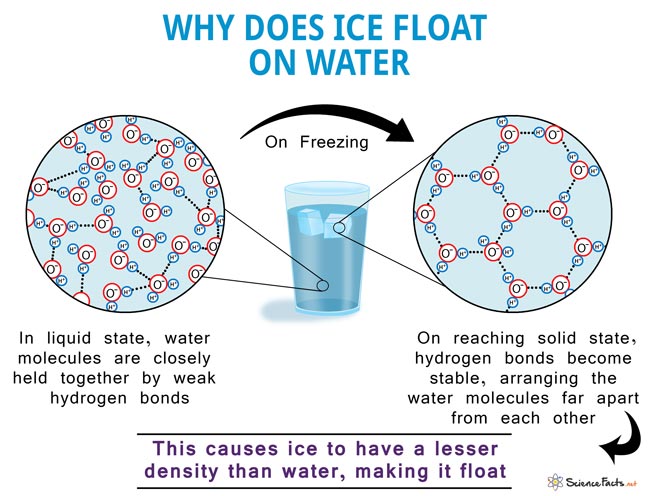
What Makes Ice Less Dense Than Liquid Water
Understanding the Reason Behind This Water is made up of 2 atoms of hydrogen and one of oxygen. The slightly negatively charged oxygen atom of a water molecule is attracted towards the positively charged hydrogen atoms of neighboring water molecules, giving rise to weak hydrogen bonds between them. In the liquid state, all the water molecules are loosely held together and are free to move. On cooling, the molecules try to come closer, increasing the density. But once the temperature drops below 4oC, the repelling nature of negative oxygen atoms of the neighboring molecules prevent them from coming any closer. That is when its density starts to fall. As it reaches the solid state (ice), the hydrogen bonds become stable and arrange themselves in such a way that the adjacent oxygen atoms are spread far apart from each other, leaving a lot of space between the molecules. This causes ice to have a higher volume and lesser density than water, making it float. – Alcohol has a lower density than ice, which makes it sink. 2. Does heavy water ice float or sink in ordinary water? – Heavy water ice sinks in regular water as it has a higher density (but it will float in heavy water).