History and Discovery of the Weak Nuclear Force The weak nuclear force was predicted by physicists Steven Weinberg, Abdus Salam, and Sheldon Glashow in 1972 and discovered in 1983 at CERN, Geneva.
Strength and Range of the Weak Nuclear Force
What does the Weak Nuclear Force do?
Properties of Weak Nuclear Force
Examples of Weak Nuclear Force
The theory describing its behavior of weak interaction and effects is often called quantum flavourdynamics (QFD). Particles interact through this weak interaction by exchanging force carriers, known as W+, W–, and Z bosons. They are known as intermediate vector bosons.
Exchange force – neither attractive nor repulsiveShort-range (~10-18 m)Non-central and non-conservativeDoes not follow product law and the inverse-square lawA million times weaker than the strong nuclear force
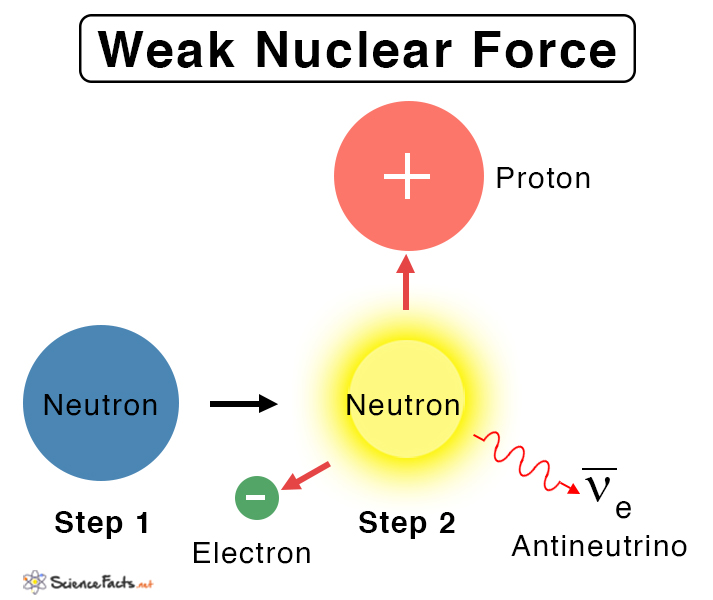
An example of a weak nuclear force is converting a neutron into a proton and vice versa, resulting in beta (minus) decay. During this process, a neutron is replaced by a proton, an electron, and an antineutrino. This equation is represented by, 0n1 → 1p1 + -1β0 + νe (antineutrino) Similarly, a proton decays into a neutron, a positron, and a neutrino in the following way, 1p1 → 0n1 + +1β0 + νe (neutrino) 2. Electron Capture Electron capture, or K-capture, is the process through which protons transform into neutrons. This reaction occurs when there is an excess number of protons relative to neutrons. An electron from the inner shell falls into the nucleus. The products are the daughter nucleus, with one less atomic number and a neutrino. An example of electron capture is the decay of krypton-81 into bromine-81. 81Kr36 + e– → 81Br35 + νe 3. Nuclear Fusion In a nuclear fusion, two protons are smashed together with enough energy to overcome the electrostatic repulsion, which results in an unstable 2He. One of the protons undergoes beta decay mediated by the weak force. A series of reactions follows, involving the formation and fusion of the intermediate 3He into a stable 4He nucleus. The overall reaction is, 4 1p1+ 2 e– → 4He + 2 νe