How Much Does Water Expand When It Freezes?
Why? – The Cause behind the Effect
How Does Water Expand When It Freezes?
Is Water The Only Substance That Expands When It Freezes?
Video
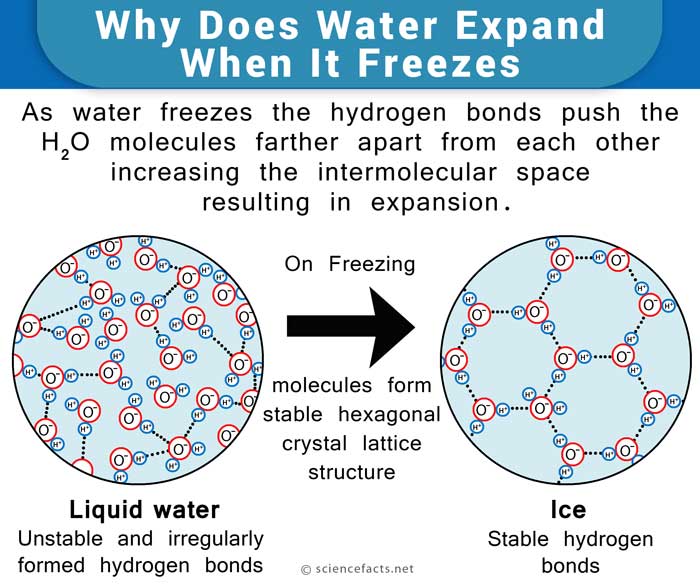
The Molecular Standpoint
The water molecule, consisting of 2 atoms of hydrogen and one of oxygen, forms a Mickey Mouse head-like structure such that the ears are the hydrogen atoms and the oxygen atom represents the head. The oxygen atom side of the molecule is slightly negative, while the hydrogen atoms side has a slightly positive charge. This makes the water molecules drawn towards each other, forming hydrogen bonds. Upon freezing, the molecules set themselves in a very open arrangement that contains more space than the water in the liquid state. Hence, water is said to expand upon freezing and becomes less dense. On the other hand, it contracts on thawing, much unlike most other liquids. This phenomenon, also known as the anomalous expansion of water, is why water bottles crack when water freezes inside. Another effect of this is the floating of ice cubes in water. Ice is of lesser density than water due to the abovementioned reason.