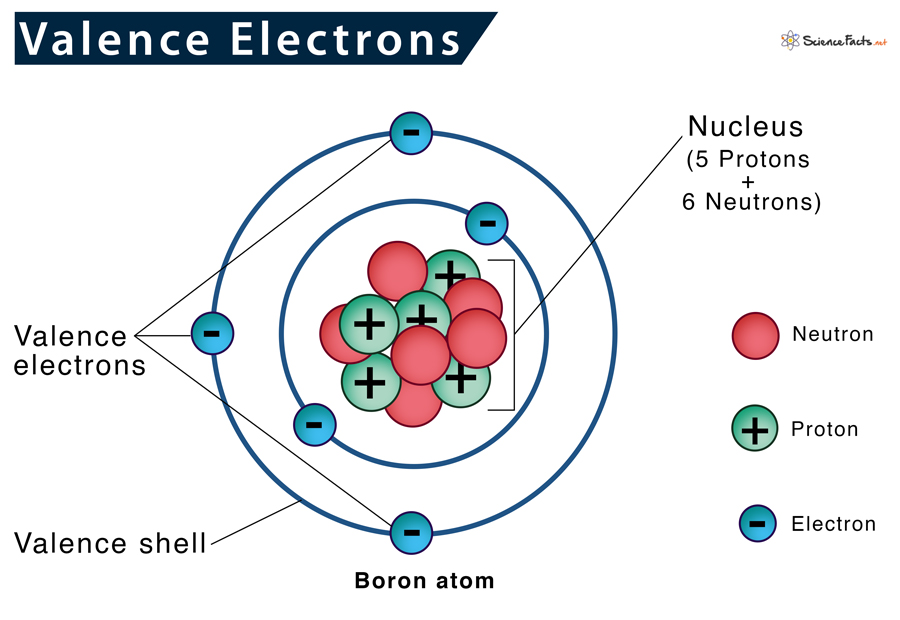
Why are Valence Electrons Important
Characteristics of Valence Electrons
How to Find Valence Electrons in an Element
Representing Valence Electrons
Atoms are most stable if they possess a filled valence shell, with each shell having 8 valence electrons. An exception to this phenomenon is hydrogen and helium, which become stable with 2 electrons in their outermost shell.
1. By Using Periodic Table
It is the most widely used method to determine the number of valence electrons in an element. Here, we just refer to the periodic table and search for the position of the element in it. As we proceed downwards in a group, the numbers of valence electrons are same, although the number of shells increases. In contrast, the number of valence electrons across a period increases by one as we move left to right of a period. Exception: As discussed, the period number indicates the number of shells, whereas the group number specifies the valence electron number in the outermost shell of an atom. However, this only holds for the main group elements, groups 1-2 and 13-18. The rule applies to the transition and inner transition elements in groups 3-12. Valence Electrons Periodic Table Chart The table below depicts the number of valence electrons in the different groups of the periodic table:
2. By Using Electronic Configuration of the Element
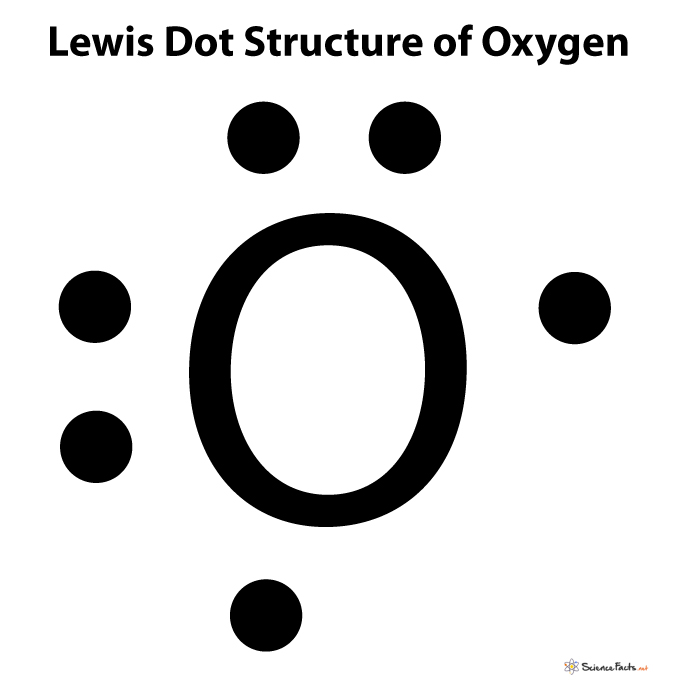
Within the shells, electrons occupy a special place called atomic orbitals. The arrangement of electrons in such orbitals is known as electronic configuration. The configuration of electrons gives a quick overview of the number of electrons present in the last shell. So, just by writing the electronic configuration of that element, we can quickly determine its number of valence electrons. Here is a table representing the number of valence electrons of elements belonging to the second period and their electronic configuration. First, write the element’s chemical symbol, and then show its valence electrons surrounding it using dots. Show only the electrons residing in the valence shell as it solely determines the chemical reactivity of an atom. For example, the Lewis symbol of oxygen depicts a ‘O’ surrounded by 6 valence electrons because oxygen has an electron configuration of 1s22s22p4.