Water moves in or out of the cell based on the solution’s solute concentration in which the cell remains suspended. Osmosis is the process that defines water’s net movement across a semipermeable membrane from a low solute concentration region to a high concentration region.
What is Tonicity
Tonicity and Osmolarity
Types of Tonicity in Solution
Examples of Tonicity in Living Systems
When solutions of two different osmolarities are separated by a selectively permeable membrane, which is permeable to water but not to solutes, water moves from the side of the membrane with low osmolarity to the side with high osmolarity. In other words, osmolarity lays down the direction of water flow in the cell membrane.
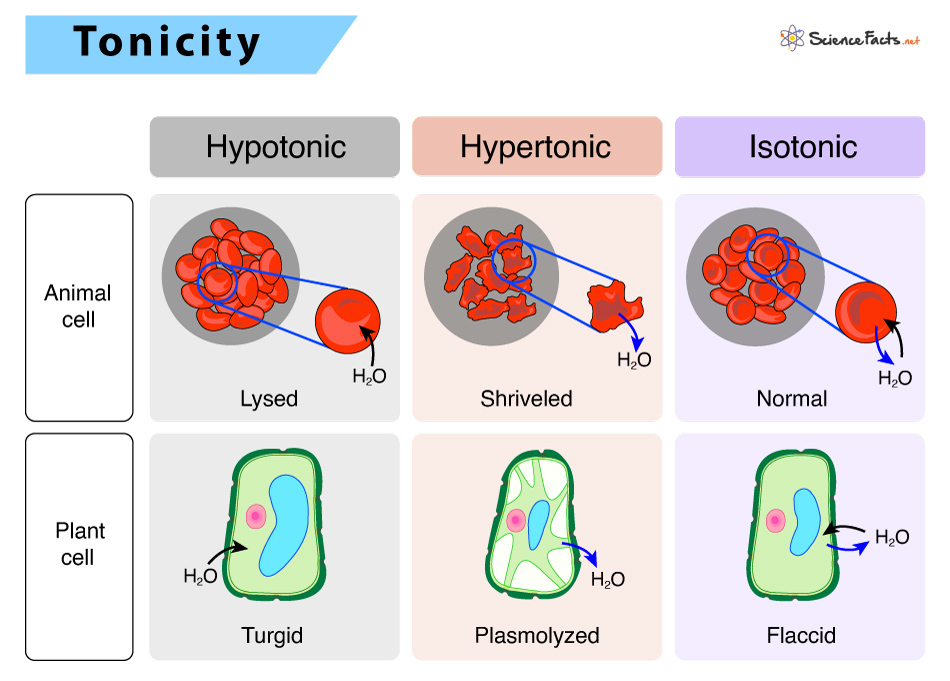
1) Hypotonic Solutions
In a hypotonic environment, the extracellular fluid has lower osmolarity or solute concentration than the cell’s fluid. In living systems, the cytoplasm is the point of reference. The prefix ‘hypo’ refers to the solution outside the cell, which has a low osmolarity than the cell cytoplasm. Thus, the extracellular fluid has a higher concentration of water than inside the cell. If a cell is placed in a hypotonic solution (example: tap water), there will be a net flow of water inside the cell from the extracellular environment, causing the cell to increase in volume or expand.
2) Hypertonic Solutions
In a hypertonic situation, the prefix ‘hyper’ refers to the solution outside the cell, which has a high osmolarity compared to the cell cytoplasm. Thus, the extracellular fluid has a higher concentration of solute than inside the cell. If a cell is placed in a hypertonic solution (example: seawater), there will be a net flow of water outside the cell, causing the cell to lose volume or shrink.
3) Isotonic Solutions
In an isotonic environment, the extracellular fluid has the same osmolarity as inside the cell. When a cell is placed in an isotonic solution, there will be no net movement of water in or out of the cell, and the cell volume will remain the same. In other words, the amount of water that moves inside the cell is equal to the amount that moves out.
In Plants
Here, the hypotonic environment is ideal for a cell to function. For the cell (plasma) membrane to function correctly without being lysed, it expands to the required limit. The cytoplasm in plants is generally slightly hypertonic to the extracellular environment, allowing water to enter the cell until its internal turgor pressure prevents further influx. When a plant is not watered, the extracellular fluid becomes hypertonic, causing water to leave the plant cell. It results in a loss of turgor pressure, causing wilting. In a hypertonic environment, the cell membrane detaches from the cell wall, causing the cell to constrict, a state known as plasmolysis. Organisms such as paramecium and amoebae, lacking a cell wall, possess specialized structures called contractile vacuoles that maintain osmotic pressure within the cell. It collects excess water from the cell and pumps it out, protecting the cell from lysis.