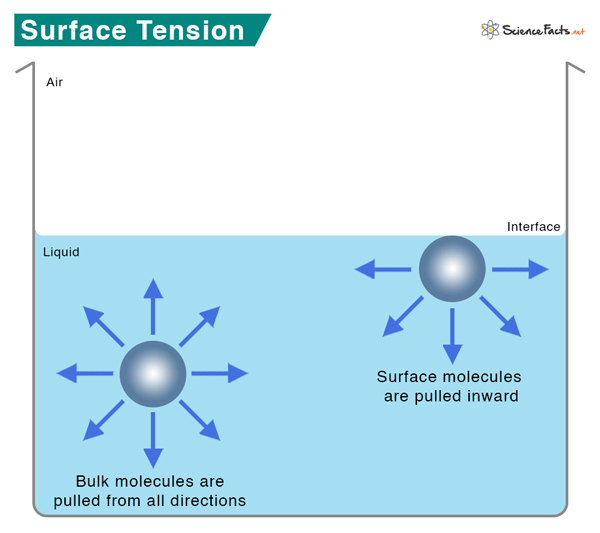
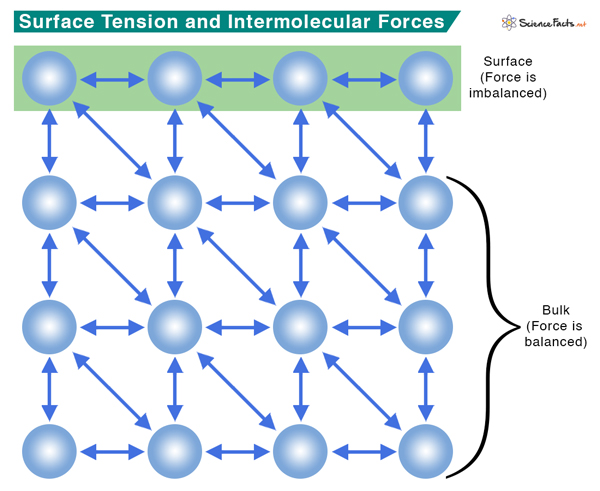
What Causes Surface Tension
Surface Tension of Water
Cohesive and Adhesive Forces
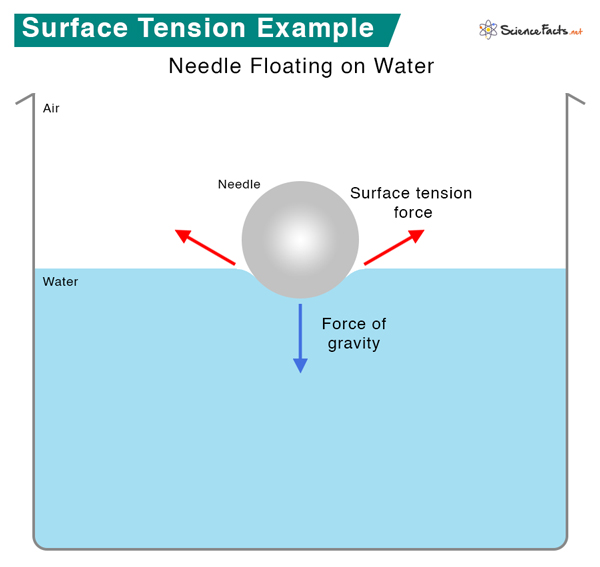
Examples of Surface Tension
Surface Tension Values
Units Since surface tension refers to the interactive force, it is quantified as the force per unit length or energy per unit area. Therefore, its SI unit is Newton per meter of N/m. Its cgs unit is dyne/cm. In terms of energy units, they are Joules per square meter (J/m2) in the SI system and ergs/cm2 in the cgs system. The shape of water and mercury meniscuses in a test tube depends on cohesive and adhesive forces. The meniscus of water is upward or concave because water wets the surface and creeps up the side. On the other hand, the meniscus of mercury is downward or concave because mercury does not wet glass. The cohesive force is strong enough to form a drop. 2. Jaundice test – Powdered sulfur is spread over urine. It will remain on the surface if urine is free of bile. However, bile reduces the surface tension of urine. If bile is present in urine, the sulfur powder will sink. 3. Hot water, soaps, and detergents – Hot water has a lower surface tension than cold water. Therefore, it is an excellent “wetting” agent. Soaps and detergents further reduce surface tension so water can enter pores and soiled parts. Therefore, detergents added to hot water make them perfect for washing clothes. 4. Liquid droplets – They are round because the cohesive forces of the surface layer pull into a spherical shape. For this reason, surface tension is an important property to study. 5. Surface tension disinfectants – Disinfectants are usually solutions of low surface tension. Therefore, they can spread out on the cell walls of bacteria and disrupt them. 6. Tents – Tents used for camping are made of materials that prevent rainwater from penetrating inside. The surface tension of water bridges the pores in the finely woven material. However, touching the tent will break the surface tension, seeping water through the tent.