Gold Foil Experiment
History
What is the Gold Foil Experiment?
Rutherford’s Gold Foil Experiment Animation
Summary
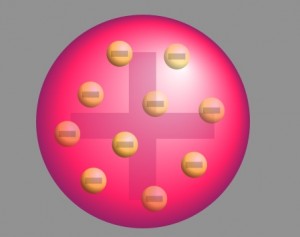
With neutrons and protons yet to be discovered, the theory was derived following the classical Newtonian Physics. However, in the absence of experimental proof, this approach lacked proper acceptance by the scientific community. Like the plum pudding model, since the positive charge of atoms was evenly distributed and too small as compared to that of the alpha particles, the deflection of the particulate matter was predicted to be less than a small fraction of a degree.
Observation
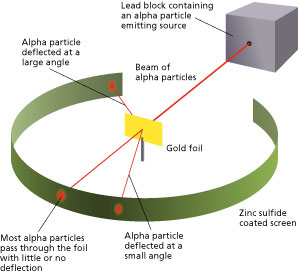
Though most of the alpha particles behaved as expected, there was a noticeable fraction of particles that got scattered by angles greater than 90 degrees. There were about 1 in every 2000 particles that got scattered by a full 180 degree, i.e., they retraced their path after hitting the gold foil.
Simulation of Rutherford’s Gold Foil Experiment Courtesy: University of Colorado Boulder
Conclusion
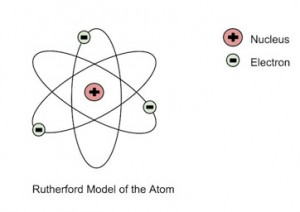
The unexpected outcome could have only one explanation – a highly concentrated positive charge at the center of an atom that caused an electrostatic repulsion of the particles strong enough to bounce them back to their source. The particles that got deflected by huge angles passed close to the said concentrated mass. Most of the particles moved undeviated as there was no obstruction to their path, proving that the majority of an atom is empty. In addition to the above, Rutherford concluded that since the central core could deflect the dense alpha particles, it shows that almost the entire mass of the atom is concentrated there. Rutherford named it the “nucleus” after experimenting with various gases. He also used materials other than gold for the foil, though the gold foil version gained the most popularity. He further went on to reject the plum pudding model and developed a new atomic structure called the planetary model. In this model, a vastly empty atom holds a tiny nucleus at the center surrounded by a cloud of electrons. As a result of his gold foil experiment, Rutherford’s atomic theory holds good even today.
Rutherford’s Atomic Model