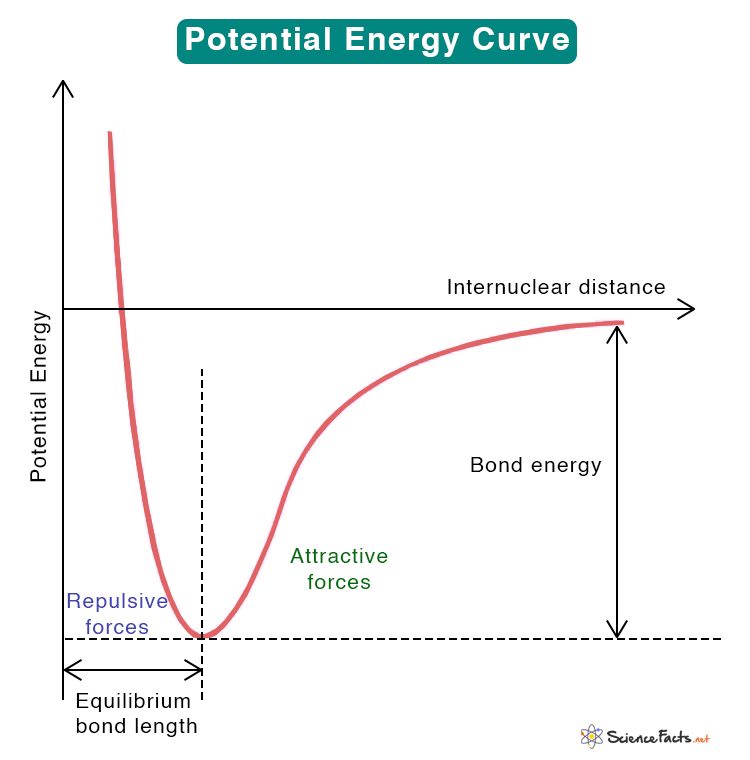
Potential Energy vs. Internuclear Distance Curve
The curve above shows the energy profile between the two atoms. The vertical axis shows the potential energy, and the horizontal is the internuclear distance, i.e., the separation between the nuclei of the two atoms. The attractive and repulsive forces balance each other where the potential energy is minimum. The internuclear distance at this minimum point is called the equilibrium bond length. Both the equilibrium bond length and the bond energy are depicted in the image.