What is Photochemical Smog
Components of Photochemical Smog
What Causes Photochemical Smog and What are the Conditions
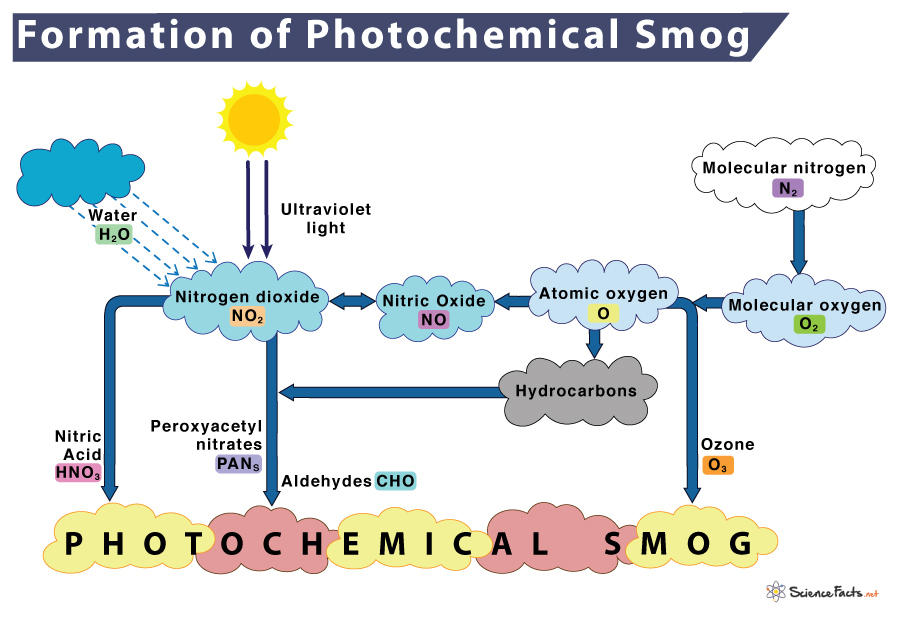
How is Photochemical Smog Formed
Why is Photochemical Smog Bad: Its Harmful Effects
How to Prevent or Reduce Photochemical Smog
It is characteristic of urban areas and is thus commonly found in densely populated cities such as Los Angeles, Sydney, Mexico, New Delhi, and Beijing, among many others. It is also sometimes referred to as ‘Los Angeles Smog.’ During morning peak traffic hours, nitrogen oxides and VOCs are hugely released into the environment. Natural calamities such as volcanic eruptions, forest fires, and microbial activities also contribute to NO and NO2. When these primary pollutants are exposed to the sun’s ultraviolet radiation, NO2 goes through a series of complex reactions with hydrocarbons to form more secondary pollutants such as ozone, peroxyacetyl nitrate, nitric acid, aldehydes, and others. The combination of all these chemicals causes the formation of photochemical smog.
- In the morning, nitrogen is burned or oxidized when released from automobiles to form nitric oxide in an oxidation step. N2 (Nitrogen) + O2 (Oxygen) → 2NO (Nitric oxide)
- Within a few hours, nitric oxide (NO) combines with more oxygen in another oxidation reaction to form nitrogen dioxide. 2NO + O2 (Oxygen) → 2NO2 (Nitrogen dioxide)
- Nitrogen dioxide absorbs sunlight to breaks down, forming nitric oxide (NO) and oxygen radical (O) in a reduction reaction. NO2 (Nitrogen dioxide) + Sunlight → NO (Nitric oxide) + O (Oxygen radical)
- Oxygen radicals react to atmospheric oxygen (O2) to form ground-level ozone (O3). O (Oxygen radical) + O2 (Atmospheric oxygen) → O3 (Ozone)
- Ozone is consumed by nitric oxide to produce nitrogen dioxide and oxygen. This reaction is reversible. O3 (Ozone) + NO (Nitric oxide) ⇌ NO2 (Nitrogen dioxide) + O2 (Oxygen)
- Nitrogen dioxide reacts with volatile organic compounds (VOCs) such as hydrocarbons (R) to form secondary pollutants such as peroxyacetyl nitrate (PAN). NO2 (Nitrogen dioxide) + VOCs (Hydrocarbons) → PAN (Peroxyacetyl nitrate) + Others
Reduce dependence on vehicles.Use public transportation instead of driving.Reduce dependence on nonrenewable energy resources that are the primary sources of air pollution.Install solar panels to trap solar energy that acts as an alternative source of energy.Use smog detectors and monitoring systems that act as early warning systems for air pollution.