Ozone depletion is defined as the gradual thinning of the ozone layer present in the lower portion of the earth’s stratosphere. When Was It First Identified In 1974, Mario Molina and Sherwood Rowland recognized the threats of the ozone layer from compounds such as chlorofluorocarbons (CFCs). Their work received validation on May 11, 1985, when three English scientists discovered a hole in the ozone layer over Antarctica. Their discovery is commonly known as the ‘ozone hole’ since the ozone depletion is not uniform. Later, ozone depletion was found to occur rapidly at the poles and, to some extent, the equator.
What Causes Ozone Layer Depletion
How is Ozone Layer Depleted: Mechanism and Equations
Harmful Effects
Solutions and Control Measures
Summary and Conclusion
The two primary sources of ozone depletion are:
- Man-made Sources It is responsible for almost 90% of the total depletion. It occurs mainly due to the excessive release of chlorine and bromine from ozone-depleting substances (ODS) such as chlorofluorocarbons (CFCs), halons, carbon tetrachloride (CCl4), hydrochlorofluorocarbons (HCFCs), and methyl bromide (CH3Br). Here is a list of some main ozone-depleting compounds, along with their sources:
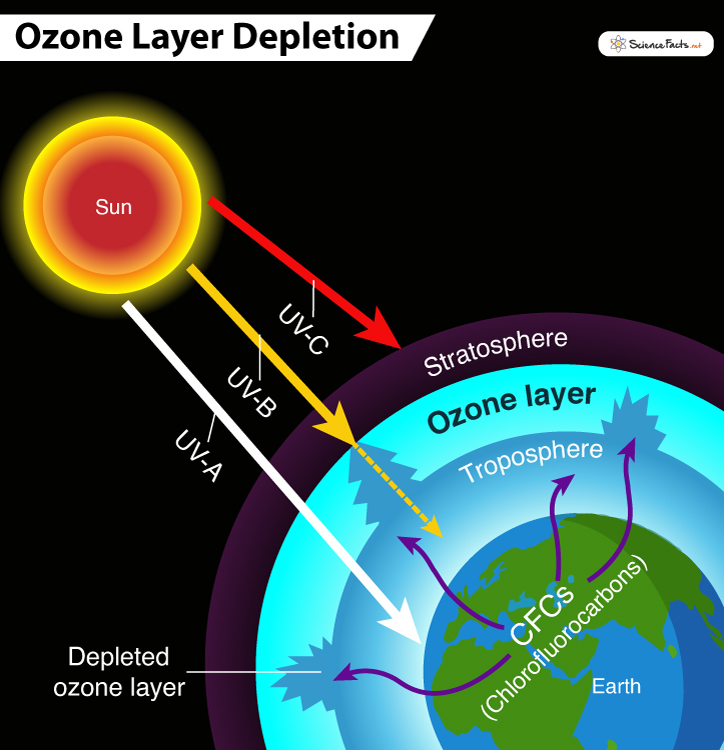
- Natural Sources Sun-spots and stratospheric winds are the two major natural causes of ozone depletion. Together, they contribute not more than 1-2% of the total depletion of the ozone layer. Their effect is temporary. CFCs are broken up by ultraviolet radiations (UV-A, UV-B, AND UV-C) from the sun releasing chlorine. Once the chlorine is released, it reacts with ozone (O3) to form chlorine monoxide (ClO) and oxygen (O2). Cl + O3 = ClO + O2 When a molecule of chlorine monoxide (ClO) meets an oxygen atom, it breaks up, releasing chlorine. ClO + O = Cl + O2 The free radical of chlorine again reacts with other ozone molecules. It destroys its structure, thus producing a catalytic cycle of chlorine. Based on the Environmental Protection Agency (EPA) report, one chlorine atom can destroy more than 100,000 ozone molecules, thus destroying them more quickly than produced naturally. Later it was discovered that bromine and specific bromine-containing molecules such as bromine monoxide were almost 40 times more potent than chlorine at destroying ozone.
Damage to Human Heath: If the ozone layer is completely depleted, humans will get directly exposed to intense UV light from the sun. Overexposure to UV rays is responsible for skin cancer, cataracts, sunburns, immune deficiency disorders, and rapid aging.Affecting Growth of Plants: Many crops such as barley, wheat, corn, oats, rice, tomatoes, and cauliflower are vulnerable to intense UV light, causing a reduction in their growth rate, flowering, and photosynthesis. Skin and Eye Cancer in Animals: Like humans, animals exposed to UV light for an extended period can cause skin and eye cancer.Threat to Marine Life: The growth of certain marine planktons decreases when exposed to high UV light. Since planktons appear at a higher level of the food chain, the entire marine ecosystem gets disturbed. Also, severe reproductive defects are found in the fishes, thus decreasing their population.Causing Chemical Degradation: Man-made chemicals such as plastics, wood, fabrics, and rubber get hugely degraded on exposure to UV light.
Some of the crucial ways to prevent ozone layer depletion are listed below:Replacing halon-based fire extinguishers with other foam-based formsDiscouraging to buy refrigerators and air conditioners that use CFCs as a refrigerantServicing old electrical equipment like air conditioners and air coolers reduces the release of chlorofluorocarbons.Discouraging the use of cleaning solvents containing CFCs or ammonia and encouraging natural and eco-friendly cleaning productsMinimizing the use of personal vehicles and using public transport reduces greenhouse gases that deplete the ozone layer.Prohibiting the use of air pollutants such as nitrous oxide that decompose ozone into nitrogen dioxide (NO2) and oxygen gas (O2)Avoid the use of fossil fuels and increasing dependence on renewable sources of energy.