Why is it Called ‘Oxidative’ Phosphorylation The process is named so, as the conversion of ADP to ATP by adding a phosphate group, is dependent on the oxidative reactions that take place in the mitochondria. The energy released by nutrients during these oxidation reactions is utilized to generate ATP through ETC.
Where Does It Take Place
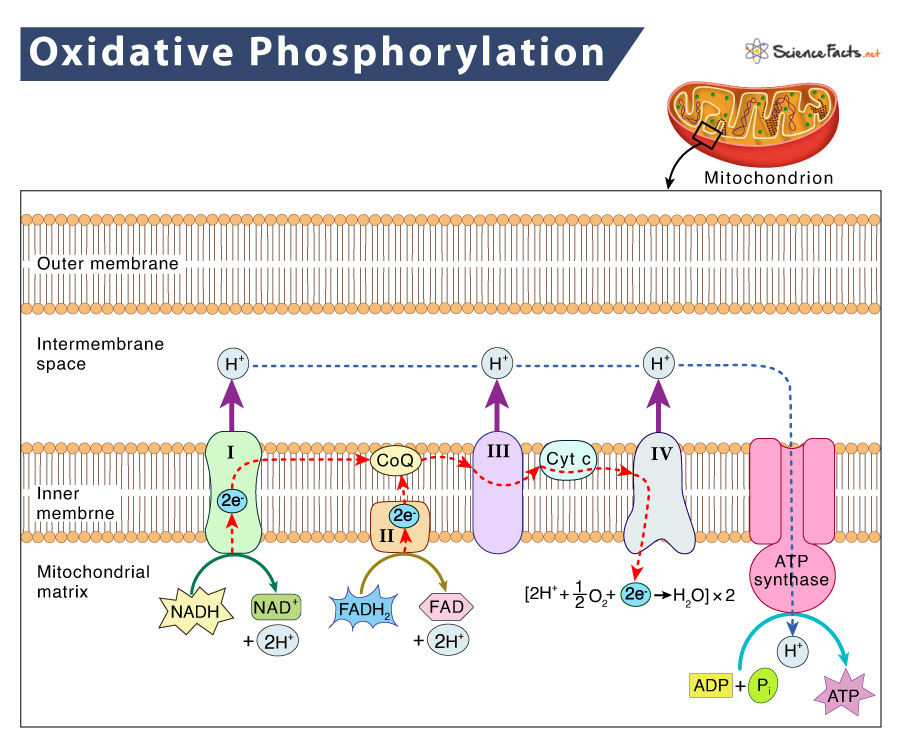
Its Steps
Total ATP Yield: How Many ATPs are Produced in Oxidative Phosphorylation
What is the Purpose of Oxidative Phosphorylation
The key steps of this process are as follows: Step 1: Delivery of electrons by NADH and FADH2 In this step, the NADH and FADH2 made in the previous phases of cellular respiration get transformed into NAD+ and FAD, respectively, by donating their electrons in the electron transport chain (ETC). Step 2: Electron transfer and proton pumping Next, the electrons donated in the previous step are passed from one member of the transport chain to another in a series of redox reactions via complex I, II, III, IV, coenzyme Q (CoQ), and cytochrome C (Cyt C). As these electrons move from a higher to a lower energy level, energy is released. Some of this released energy is utilized to pump H+ ions or protons out of the mitochondrial matrix, into the intermembrane space, creating a proton gradient. Step 3: Splitting of oxygen to form water At the end of the ETC, electrons are transferred to the oxygen molecule, which splits into half and takes up H+ to form water. Step 4: ATP synthesis As the H+ ions flow down their gradient, getting back into the matrix, they pass through an enzyme called ATP synthase, forming ATP. This enzyme controls the flow of protons to synthesize ATP. As a whole, we can observe that oxidative phosphorylation uses the chemical reactions that release energy to drive another chemical reaction that requires energy. In simple terms, first, it uses the energy released by ETC to form an electrochemical gradient, and then uses this energy stored in the gradient to produce ATP. This process, in which energy from a proton gradient is used to make ATP, is called chemiosmosis. These two sets of reactions, ETC and chemiosmosis are coupled, as one cannot occur without the other. Note: There are some chemical substances that make the inner mitochondrial membrane permeable to protons. Due to this, the protons directly leak back into the mitochondrial matrix, rather than through the ATP synthase. As a result, the link between ETC and chemiosmosis gets disrupted, disturbing the whole process. This kind of reaction is called an uncoupling reaction. Dinitrophenol (DNP) is such an uncoupling agent or uncoupler.
The Equation
NADH + FADH2 + O2 + ADP + Pi → NAD+ + FAD + H2O + ATP Reactants Products