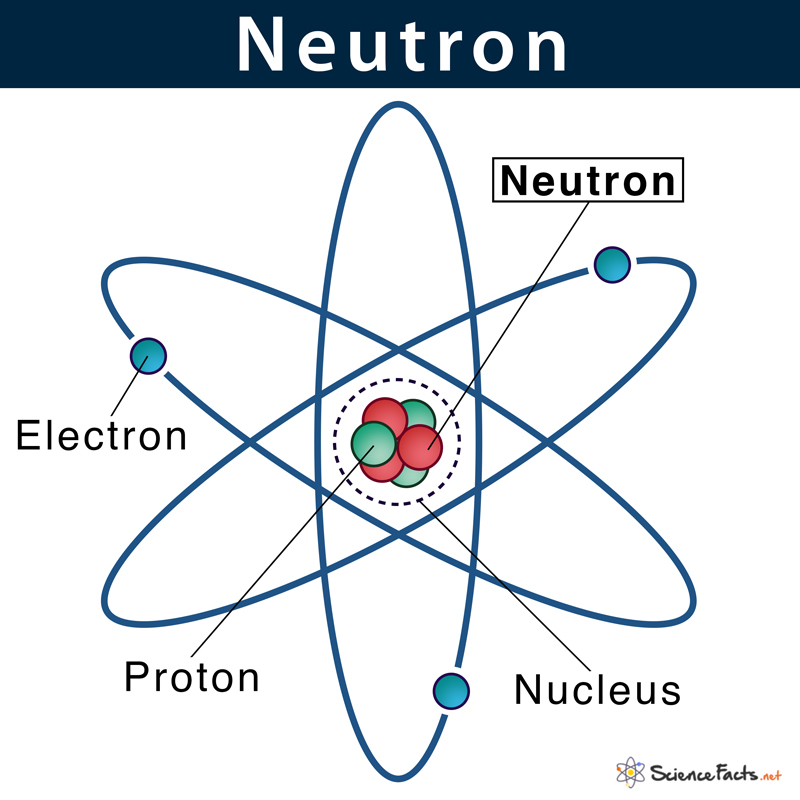
The word ‘neutron’ was derived from the Latin word ‘neuter’, meaning neither, and the Greek suffix ‘on’ is consistent with the names of all sub-atomic particles, including proton and electron.
Who Discovered Neutron
Where are they Located
What are Neutrons Made of
Characteristics
- Charge: Unlike proton and electron, the neutron has no charge. In other words, they are electrically neutral and thus are represented as n0. Here, ‘0’ stands for zero charges.
- Mass: It has a mass equal to one atomic mass unit (u) or 1.675 x 10-24 g. This is slightly greater than the mass of a proton and roughly 1,836 times the mass of an electron. Together with protons, they make up virtually all of the mass of an atom.
- Number in Atoms: Generally, all atoms have the same number of neutrons as protons within the nucleus. For example, the number of neutrons in the element Osmium is 114.
- Movement: They travel in straight lines and produce shadow of the objects placed in their path.
- Other: The number of neutrons may vary for the atoms of the same element. Atoms of the same element that vary in their numbers of neutrons are called isotopes. For example, 99% of carbon atoms have six neutrons, while the rest 1% have seven or eight neutrons.