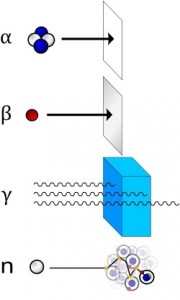
Forms of Ionizing Radiation
Types of Ionizing Radiation
Ionizing and Non-Ionizing Radiation
Sources of Ionizing Radiation
Uses of Ionizing Radiation
Harmful Effects
Precautionary Measures
Directly Ionizing
Alpha Particles
Alpha particles are helium nuclei or doubly positively charged Helium atoms. Since they have a large mass, they can’t travel through a medium at great speeds. This increases their chances of a collision with other atoms on the way. That is the reason alpha rays have a high ionization power. However, due to their frequent collisions with other particles, they tend to lose energy quickly. Hence, they cannot travel great distances in a medium and are said to have very low penetrating power.
Beta Particles
Beta particles are electrons. As electrons have very little mass, they can travel at great speeds (close to the speed of light). This attribute decreases their ionization power. However, as they can travel vast distances in a medium, they are highly penetrating.
Gamma Rays
These are electromagnetic waves having high frequency but low wavelength. They have the maximum penetrating power, but their ionization power is very low.
Indirectly Ionizing
Neutrons
They have no charges on them but can cause secondary ionization. When fast-moving neutrons strike hydrogen nuclei, they transfer their energy to the protons. As a result, charged speedy protons radiate from the hydrogen nuclei which, in their turn, directly ionize other atoms.
Natural sources
Ionizing radiation has many sources in nature.
Radon
This naturally occurring element is the primary source of ionizing radiation. Due to pressure differences between the air gaps in the soil and space inside buildings, this heavy gas can seep through such gaps and accumulate indoors. Since modern houses are so constructed to allow minimum exchange of air with the atmosphere when the doors and windows are closed, radon gets trapped inside taking its exposure to the human body much above the permissible level. If they get an option to mix with outside air, they get diluted to normal acceptable levels.
Terrestrial Sources
Bedrock and soil contain deposits of uranium and thorium which can radioactively decay into products that cause ionizing radiation. A radioactive isotope of potassium present in the Earth’s crust also contributes towards the same.
Cosmic Radiation
During the course of its revolution round the sun, high energy particles and gamma rays strike the Earth. This acts as the origin of ionizing radiation on the Earth’s surface. However, they are absorbed to a large extent by the layer of atmosphere surrounding the blue planet that acts as a buffer. Hence, people residing at higher altitudes where the air is rarer, have more exposure than those staying on the plains, near the sea level. Also, people in air travel and astronauts traveling into outer space have greater cosmic exposure risk. They are also known as background radiations of the Earth.
Internal Radiation
Some radioactive isotopes like Carbon-14 and Potassium-40 naturally occur in food and drinking water and enter the human body through the digestive tract. This is mainly because crops are cultivated in soil containing these minerals which can persist in the human body and cause internal radiation exposure.
Artificial Sources
Some sources can also be man-made.
Medical Sources
It has a multitude of sources in medical and dental sectors, mainly in equipments used for diagnosis of diseases and in nuclear medicines.
Industrial Sources
Ionizing radiation has sources in various industries including the engineering and constructions, nuclear, non-destructive testing and oil and gas production industries. The workers in these factories can get considerable occupational exposure to radiation if not properly regulated.
Consumer Products
Some of these products naturally contain radioactive materials such as some brick and stone building materials, natural gas, phosphate fertilizers, gas mantles, certain road signs which glow in the dark, tobacco products, etc.
Atmospheric Testing
Nuclear weapons were tested in the atmosphere starting from the end of World War II up to as late as the 1980s. This released radioactive materials, collectively called fallout, in the environment. While the fallout were settling to the ground, they were absorbed in the surroundings and keep emitting ionizing radiation to this day, however, small in amount.
Medical
Traces of radioactive materials are injected into the blood stream, and their path through the body is tracked by radiation detectors to identify cancerous tumors and clogs in blood vessels. A radioactive isotope of iodine is used in injections which collects in the thyroid gland and helps in the treatment of Grave’s disease. They are also used in the X-ray machines for diagnostic purposes, as nuclear medicines for treating cancer, etc.
Industrial
Ionizing radiation is used in irradiators for sterilization of products, in shrink-wrap packaging, for measuring thickness of materials, in smoke detectors, to determine the emptiness of cans before sealing, in estimating the reserves in oil fields, in nuclear gauges used in the construction of roads, in density gauges to calculate flow of materials through pipes, for examining quality of welds in bridges and buildings, for generation of electricity from nuclear and thermal power plants, etc. Other applications include curing adhesives, resins, wires and cable jackets, tyre manufacture, treatment of waste, preservation of food, etc. The term “curing” here refers to the toughening or hardening of a polymer by electron beams, heat, chemical additives or ionizing radiation.
Education and Research
The carbon-14 isotope is used in carbon dating to determine the age of materials.
Unit
The hazardous effect on living cells is measured with the help of certain units such as gray (Gy). Gy is used to measure the total dosage of radiation received by the human body. The biological effect or the effective dose which has the potential of causing harm is measured in another unit, millisievert (mSv). The average global natural dose for humans is 2.4 mSv per year.
Biological Effect
If the entire body receives very large doses of above 5,000 mSv within a short span of time, it can be fatal. However, low doses or high doses delivered over a long period of time, causes lesser damage as it is more likely for the affected cells to repair themselves successfully. Still, some cells might get genetically mutated resulting in long-term effects such as cancer. Beyond a certain limit, exposure can cause skin redness, radiation burns, hair loss, acute radiation syndrome or even impair the functions of some tissues and organs. The severity of these symptoms increases with higher dosage. Doses above 100 mSv pose considerable risks of having cancer.
Effect on Pregnancy
Amounts over 100 mSv, when administered to a pregnant woman might cause brain damage to the foetus if it is aged between 8 to 25 weeks, not otherwise. Nevertheless, X-rays are not recommended as a precautionary measure. An ultrasound scan can be a better alternative in this regard. The products which do not have any potential danger are labeled with the following symbol. The dangerous sources capable of death or serious injury have been labeled the following by the International Atomic Energy Agency. While we can do little to reduce the natural sources of radiation, artificial sources can be controlled to a great extent. A proper balance should be maintained between the beneficial and adverse effects for the benefit of mankind and advancement of the society.