IFs are dynamic, motile elements that interact with a range of cellular proteins to function. They are the most stable cytoskeletal component and thus provide mechanical strength to cells and tissues.
Where are Intermediate Filaments Found
Structure
Functions
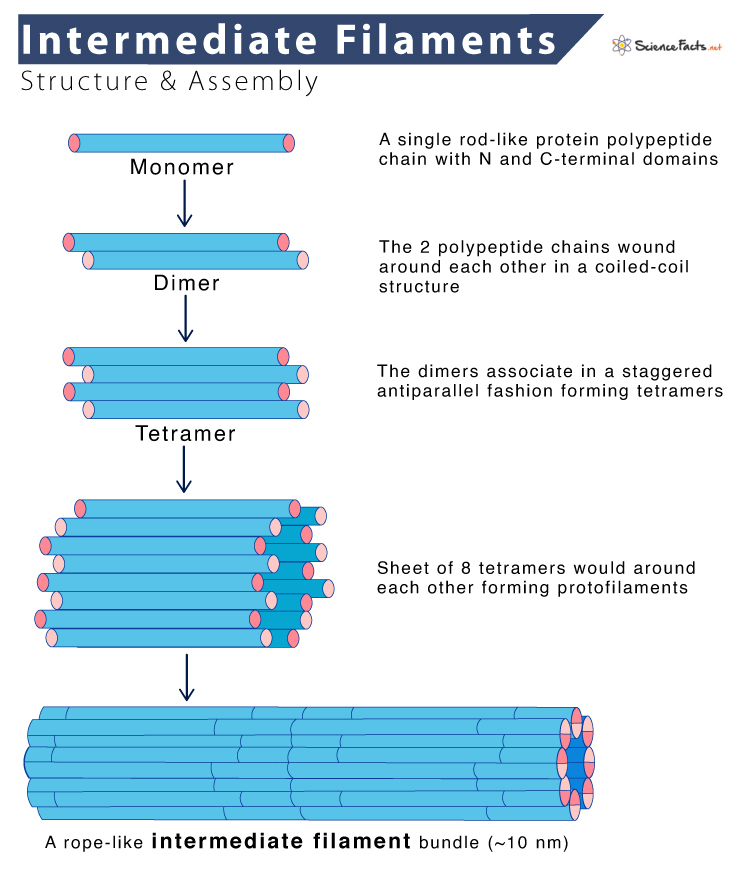
Despite considerable differences in size and amino acids sequence, the various intermediate filament proteins share a typical structural organization. Each IFs monomer consists of a central α-helical rod domain of approximately 310 amino acids, flanked by amino- and carboxy-terminal domains. The central domain varies among intermediate filament proteins in size, sequence, and secondary structure.
Types of Intermediate Filament Proteins
IFs are the most diverse group among all cytoskeletal elements made of various proteins expressed in different types of cells. At least 50 different intermediate filament proteins have been identified so far. They are classified into different groups based on similarities between their amino acid sequences. They are:
Intermediate Filaments Assembly
Thus, in contrast to actin filaments and microtubules, intermediate filaments do not have distinct plus and minus ends and thus lack polarity.
Regulating cell shape in association with some other proteins such as cadherins and integrinAnchoring the nucleus and other organelles in place and thus creating supportive scaffolding inside the cellIntegrating the cytoskeletonRegulating some key signaling pathwaysMoving proteins to specific domains of polarized cells such as Sertoli cellsProviding high tensile strength in durable structures such as hair, scales, and fingernailsMaintaining the alignment of myofilaments and sarcomeres in skeletal muscles
Apart from structural functions, IFs also have a significant role in cell-type-specific functions and regulate gene expressions and mitosis.