Insulin receptors, aquaporins, cell adhesion molecules such as integrin, cadherin, selectins, rhodopsin, and G-protein coupled receptors are typical examples of integral membrane proteins.
Structure of Integral Membrane Proteins
Functions of Integral Membrane Proteins
Generally, an integral protein exhibit three different structures, having two protein and one lipid part. Structurally, they exhibit an alpha helix or a beta-barrel and contain an amphipathic lipid anchor. The different parts of an integral membrane protein are:
Alpha (α) Helix
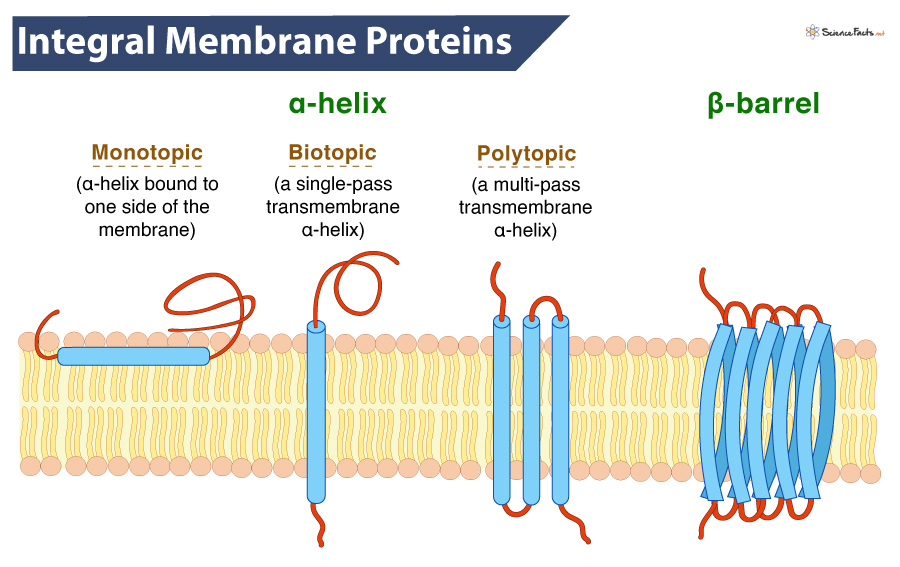
It consists of a chain of non-polar amino acids that remains bound within the hydrophobic tail region, spanning the entire membrane. It is named so because it is α-helical in shape. The alpha helix creates a protein channel or a hole in the plasma membrane, which allows various substances to pass. Depending on the relationship with the lipid bilayer, an integral protein can be: Integral monotopic proteins are associated on one side of the membrane but do not span the lipid bilayer completely. Transmembrane proteins span the lipid bilayer completely. Single-pass membrane proteins cross the membrane once (biotopic). In contrast, multi-pass membrane proteins cross the membrane more than once (polytopic). They are found in all types of biological membranes. Single-pass transmembrane proteins can be Type 1 proteins having their carboxyl-terminus towards the cytosol or Type II with their amino-terminus towards the cytosol. Type III proteins have multiple transmembrane domains in a single polypeptide chain. In contrast, type IV proteins consist of several polypeptides assembled in a channel through the membrane. Type V proteins are bound to the lipid bilayer covalently. Finally, Type VI proteins have both transmembrane domains and anchoring lipids.
Beta Barrel
If not an alpha helix, the protein consists of a folded chain of amino acids forming a flattened, rigid sheet. Many sheets extend through the membrane, creating a pore and forming a barrel shape. The outer part of the beta sheets contains hydrophobic residues, and the integral protein is locked into the plasma membrane.
Amphipathic Lipid Anchor
It is a non-polar, hydrophobic attachment found in some proteins that allows them to be embedded within the plasma membrane. It anchors to the plasma membrane with other lipids and is one of its major constituents. They also function as receptors, linkers, enzymes, signal transducers, cell adhesion, and anchorage within and outside the cell in a tissue. Some integral membrane proteins are part of large complexes of proteins that are responsible for numerous reactions that take place across a membrane. For example, ATP synthase found on the inner mitochondrial membrane is a multi-protein complex synthesizing ATP for the organism. In contrast, some integral proteins, such as neurotransmitters, are not bound to any membrane. Their products easily escape and work in the region where it is most needed.