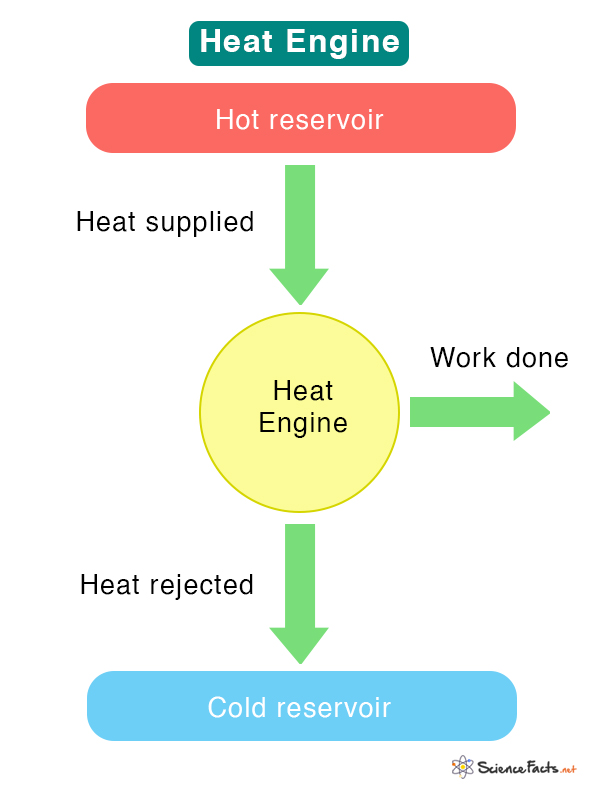
An example of a heat engine is an automobile engine. Here, the burning fuel is the hot reservoir, and the surrounding is the cold reservoir in which combustion products are exhausted.
How Does a Heat Engine Work
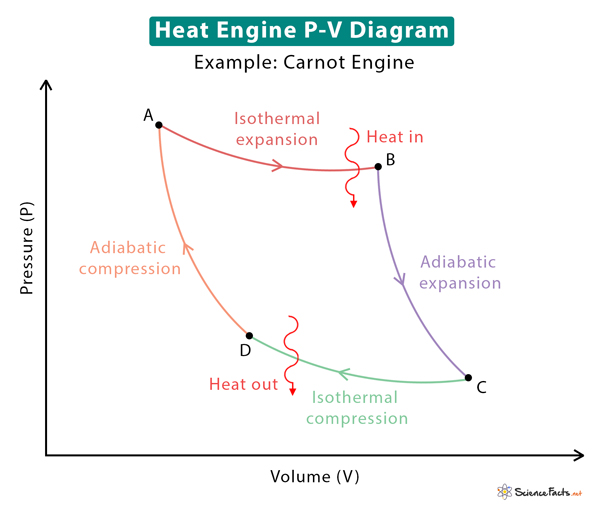
Heat Engine P-V Diagram
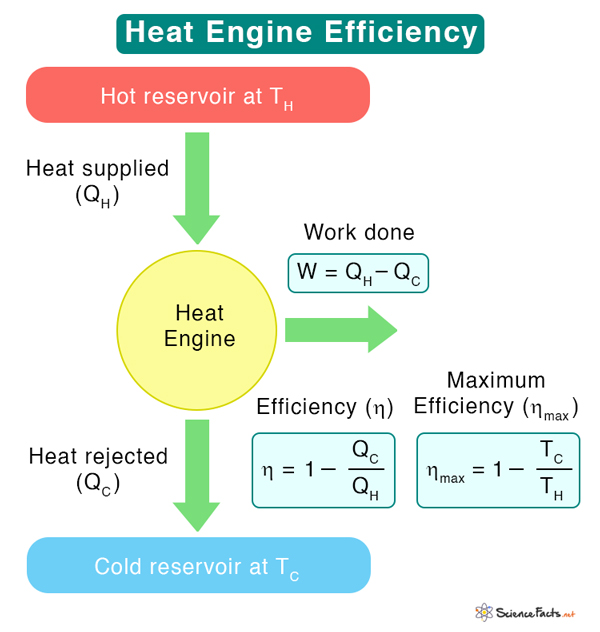
Efficiency of Heat Engine
Types and Examples of Heat Engine
The amount of work extracted from a heat engine depends upon its efficiency. Not all heat engines are 100% efficient, which means that not all heat supplied to the engine is converted into work. Some heat is lost due to friction and wear of mechanical parts, resulting in reduced efficiency. The first and second laws of thermodynamics establish a relationship between heat and work and limit the operation of a heat engine. The first law is about energy conservation, while the second law limits efficiency and determines the direction of energy flow. The P-V diagram also gives a visual representation of the work done during volume expansion and the change in internal energy during temperature changes. Recall that the internal energy depends on the temperature. The internal energy changes combined with the first law give the amount of heat absorbed and rejected by the engine. The image below shows the P-V diagram for the Carnot engine. Various stages of the Carnot cycle are indicated in the diagram. Suppose QH is the quantity of heat extracted from the hot reservoir at temperature TH, and QC is the heat dumped into the cold reservoir at temperature TC. Then, the amount of work obtained from the engine is By definition, efficiency is given by Since TH and TC are easily measurable, let us express efficiency in terms of temperatures using the second law of thermodynamics and entropy. Irreversible and Reversible Processes The change in entropy is According to the second law, entropy always increases for an irreversible process. The second law also states that the entropy change is zero for a reversible process. Which ultimately leads to the expression However, 1 – TC/TH is the efficiency of a reversible engine such as the Carnot engine. Therefore, the efficiency of a heat engine is limited by the Carnot theorem. According to this theorem, “Any system working between two given temperatures (hot and cold reservoirs) can never have an efficiency more than the Carnot engine working between the same two reservoirs.”
External Combustion Engine
In an external combustion engine, the fuel burns outside the engine’s main body, where the action takes place. The steam engine is a typical example of an external combustion engine. Here, coal is burnt to heat water, the working substance. The water is then converted into steam. The steam passes through a cylindrical pipe whose one end consists of a piston moving back and forth. The piston is connected to anything that requires motion, like locomotive wheels. This back-and-forth piston action allows the wheels to rotate and makes the train move forward.
Internal Combustion Engine
In an internal combustion engine, the fuel burns inside the engine. An automobile engine is a perfect example of an internal combustion engine. It consists of several cylinders where the gaseous fuel or gasoline continuously burns, producing heat. Thus, the fuel is the working substance. The heat causes the pistons attached to the cylinders to move back and forth. This mechanical action of the pistons drives the wheels and makes to vehicle move forward. Internal combustion engines are more efficient than external combustion engines because the fuel is combusted internally, comes in contact with the piston, and fires the engine.