Glycogenesis is the biosynthesis of glycogen, the carbohydrate stored in the liver and muscle cells, by adding glucose molecules chain-wise. It is an anabolic process that requires one ATP molecule per glucose incorporated into the glycogen. Glycogen is synthesized depending on the cell’s demand for glucose and ATP. Excess glucose is stored in glycogen if blood glucose levels and ATP concentrations are high. In animals, glycogenesis occurs in the cytoplasm of muscle, liver, and adipose tissue cells.
Process
Functions of Glycogenesis
Regulation of Glycogenesis
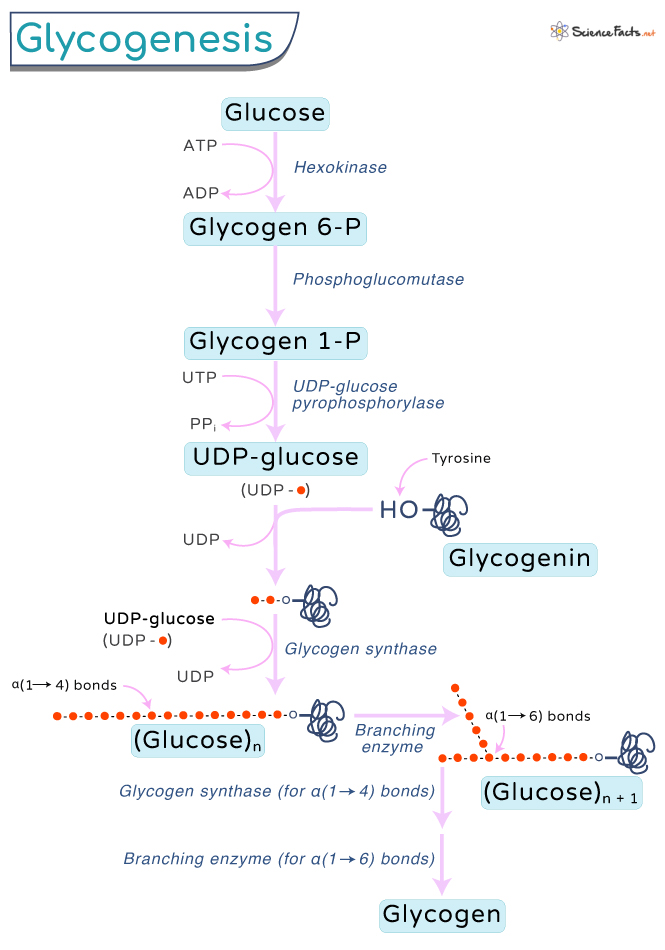
Step 1: Glucose is first converted to glucose 6-phosphate by the enzyme glucokinase or hexokinase. This step is an energy-dependent process where an ATP is used to form ADP. Step 2: Glucose-6-phosphate is then converted to glucose-1-phosphate by the enzyme phosphoglucomutase. It is a rearrangement reaction as the position of the phosphate group is changed from 6 to 1 in the glucose molecule. Step 3: Glucose-1-phosphate is then converted to UDP-glucose (glucose uracil diphosphate) by the action of the enzyme UDP-glucose pyrophosphorylase. The pyrophosphate formed is later hydrolyzed to two phosphate molecules. There are two phosphate groups in this glucose and the amino acid uracil. Step 4: Further, an enzyme called glycogenin adds UDP-glucose one after the other, forming a short, linear chain of UDP glucose. This step is repeated until approximately seven to eight glucose monomers polymerize. Glycogenin has a tyrosine residue on each subunit that serves as an anchor to UDP-glucose. The enzyme adds around seven UDP-glucose molecules to each tyrosine residue, forming α(1→4) bonds. Step 5: Once a chain of seven to eight glucose residues is added, glycogen synthase binds to the growing chain. It adds the following UDP-glucose molecule to the 4-OH group of the glucosyl residue to the glycogen chain at its non-reducing end through α(1→4) bonds. Step 6: Simultaneously, the glycogen branching enzyme transfers the end of the chain to the existing chain through the α-1:6 glycosidic bond, forming branches, which grow through the addition of more α-1,4 glycoside residue. However, when cells have depleted all their glucose, they can use glycogen as an alternative energy source. Muscle cells, for example, use glycogen during stressful work like exercising and running when the glucose reserve is exhausted. The concept of ‘carbo-loading,’ where athletes eat carbs and sugar, is also based on glycogenesis.
Helps to Conserve Water
The products of glycogenesis are less polar and more compact than glucose. Thus, unlike glucose, they do not dissolve in water. As cells can store many glucose molecules within glycogen, they will require less water to hold than when the glucose molecules are present individually.
Through Epinephrine
Epinephrine or adrenaline, as commonly called, acts as an emergency stress hormone in our body and is negatively regulated by glycogenesis. Thus, during an emergency, it stops glycogenesis and starts glycogenolysis to overcome the situation.
Through Insulin
After a heavy meal, the blood glucose levels remain high. Under such a situation, the pancreas releases insulin, which converts excess glucose to glycogen by stimulating glycogenesis and stored in the liver. It causes the cell to store glycogen and reduce the sugar present in the blood. When cells require glucose during periods between meals, blood release glucose from glycogen and keep the glucose level intact.