What are Enzymes
Are All Enzymes Proteins
Components
Structure: What are They Made of
Properties
When and How does an Enzyme Work to Catalyze Reactions
Factors Affecting Enzyme Activity
Types and Examples
Functions: Why are Enzymes Important
They are found in all living cells that vary in type based on the function it performs. Enzymes help in the process of digestion, blood clotting, and hormone production.
Cofactors
Some enzymes require a non-protein part for their functioning, known as cofactors. Cofactors are essential for the functioning of the enzyme. An enzyme devoid of a cofactor is an apoenzyme, while an enzyme with its cofactor is called a holoenzyme. There are three types of cofactors:
- Prosthetic Groups: They remain tightly bound to an enzyme all the time. Example – FAD
- Coenzyme: They bind to an enzyme only during catalysis. Example – NAD+
- Metal Ions: Certain enzymes require a metal ion at their active site for catalysis. They form a coordinate bond with the enzyme. Example – Zn2+ The protein folds upon itself when the hydrogen in the (NH2) group and the oxygen in the (COOH) group forms a hydrogen bond. It results in the folding of the protein in the 2-dimensional plane. There are two types of secondary structures – α-helix and β-sheet. As a result of folding the 2-D linear chain in the secondary structure, the protein can fold up further, which helps it gain a 3-D structure. This formation is the tertiary structure of the protein.
Protein in nature except for ribozymeActivity depends on several environmental factors such as temperature, pH, and concentration of substrate and productsInitiate chemical reaction(s)Accelerate the rate of chemical reaction(s). The rate of an enzyme-catalyzed reaction usually is (103 – 108) times faster than an uncatalyzed reaction.
Apart from the general properties, enzymes have four more essential properties. They are given below: Catalytic
Have excellent catalytic powerHighly capable; a small quantity of an enzyme can catalyze a large quantity of a specific substrates.It remains unaltered at the end of the reactionThe turnover number ranges between 0.5 to 600000
Specificity
Specific to a particular type of reaction. A particular enzyme catalyzes a particular reaction binding to a particular substrate.
Reversibility
Most reactions are reversible. The reversibility depends on the requirement of the cellIn some cases, there are separate enzymes for forwarding and backward reactionSome reactions are not reversible
Sensitivity to Temperature and pH
Highly sensitive to heat, temperature, and pHActivity is maximum within a specific range of temperature and pH ranges. Below which they have reduced activity and above which they get denatured and thus lose their activity.
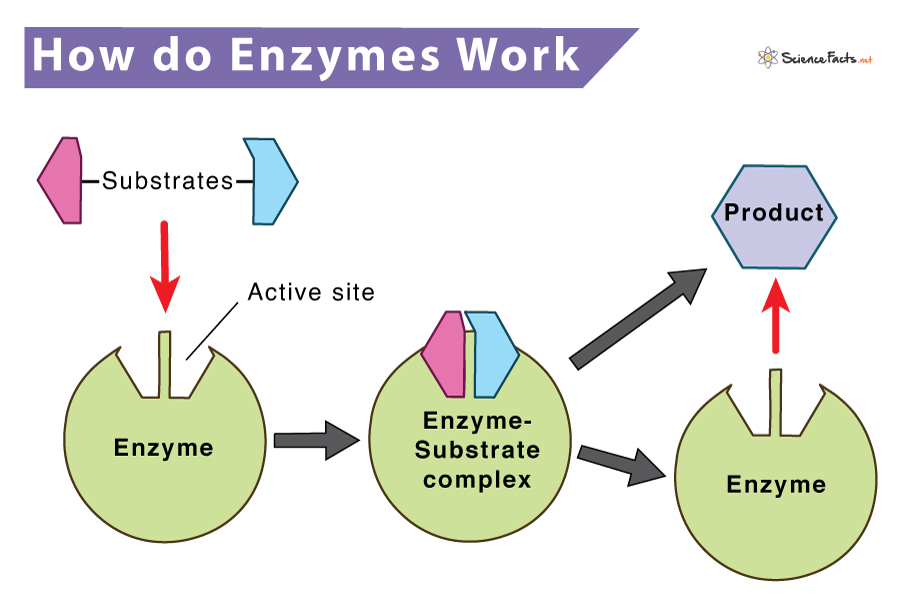
Enzymes do not change the free energy of the reactants and the products and thus do not affect a reaction’s ∆G value. Instead, they work by lowering the transition state, an intermediate state in the reaction. They also keep the equilibrium constant (Keq) same throughout the reaction. While catalyzing a chemical reaction, an enzyme binds to one or more reactant molecules. These molecules are enzymes substrates. In some reactions, one substrate molecule breaks to form multiple products. In contrast, in others, two substrate molecules join to form one large molecule. The part of the enzyme where the specific substrate binds is called the active site. The basic mechanism is divided into two steps, as shown below: Step 1: Enzyme (E) + Substrate (S) <—–> Enzyme-Substrate complex (ES) Step 2: Enzyme-Substrate complex (ES) <—–> Enzyme (E) + Product (P) The above two steps can be combined as follows to give the complete reaction. Overall Reaction: Enzyme (E) + Substrate (S) <—–> Enzyme-Substrate complex (ES) <—–> Enzyme (E) + Product (P) The amino acids present in the active site of the enzyme give the enzyme a definite shape. The shape uniquely determines its substrate and helps it to bind and form the enzyme-substrate complex. The enzyme then converts the bound substrate to a product with itself remaining chemically unchanged.
How do They Speed up Reactions
As stated above, enzymes work by decreasing the activation energy, like all catalysts. They generally lower the activation energy by reducing the energy needed for reactants to react during a reaction. Lesser the activation energy of a reaction, the faster the rate of the reaction. They do so in the following ways:
Bring the reactants together such that they do not need to expend energy moving about to collide.Position the reactants correctly so that they do not have to overcome intermolecular forces that would typically push them apartChange the pathway so that the reaction can occur by the pathway with lower activation energy.
What Happens to an Enzyme after a Biochemical Reaction
After the reaction, the products formed are released from the active site of the enzyme. The enzyme remains unaltered at the end of the reaction and thus is free to bind another substrate and catalyze a new reaction.
Temperature: Increasing temperature increases the reaction rate, and lowering a temperature slows down a reaction. However, extremely high temperatures can cause the enzyme to denature and lose its functional activity. There is a specific temperature at which the enzyme activity is at its greatest. The optimum temperature is around 35.5°C for the enzymes in human cells.
pH: Each enzyme works best within a particular range of pH. Beyond that, it is found to have reduced activity. An extremely high temperature can cause the enzyme to denature
Enzyme Concentration: Increasing enzyme concentration speeds up a reaction until there are available substrates to bind. Once all the substrates get bound to their enzymes, the reaction shall no longer increase.
Substrate Concentration: Increasing substrate concentration also increases the reaction rate to a certain point. Once all the enzymes get bound to their substrates, a further increase in the substrate concentration will not affect the reaction rate.
Apart from the above factors, enzyme activity is also affected by ion concentration and the presence or absence of activators and inhibitors. Thus, enzymes work best within a specific range of temperature and pH ranges. Animals, including humans, have a vast number of enzymes working inside the human body. They are widely grouped into metabolic, digestive, and food enzymes.
Metabolic enzymes help produce energy and detoxify them by breaking down food particles consisting of protein, fat, and carbohydrates.
Examples are carboxylases, dehydrogenases, oxidoreductases, kinases, lyases, transferases, and many more.
Digestive enzymes catalyze reactions that break down macromolecules – carbohydrates, proteins, and fats into smaller molecules that help the body to produce energy.
Examples are amylase, lipase, maltase, peptidase, and protease.
Food enzymes are not naturally found in the body of living organisms, but we get their benefit from the food and their supplements.
Examples are cellulase, papain, actinidin, bromelain, and ficin. Listed below are some more enzymes present in our body with their purpose:
Lactase: Breaks down lactose, the complex milk sugarPectinase: Breaks down pectin found in fruits and vegetablesCatalase: Breaks down hydrogen peroxide into water and oxygenDNA polymerase: Synthesize DNA from deoxyribonucleotidesTrypsin: Breaks down protein into amino acidsAcetylcholinesterase: Breaks down the neurotransmitter acetylcholine
Enzymes are also needed in industry and household products. They are also commercially used to produce fermented products such as beer, wine, and cheese. In the clothing industry, enzymes play a role in reducing impurities in cotton.