The laws of electrostatics were discovered by French physicist Charles Augustin de Coulomb in 1785 and are known as Coulomb’s law.
Properties of Electrostatic Force
Examples of Electrostatic Force in Daily Life
Applications of Electrostatic Force
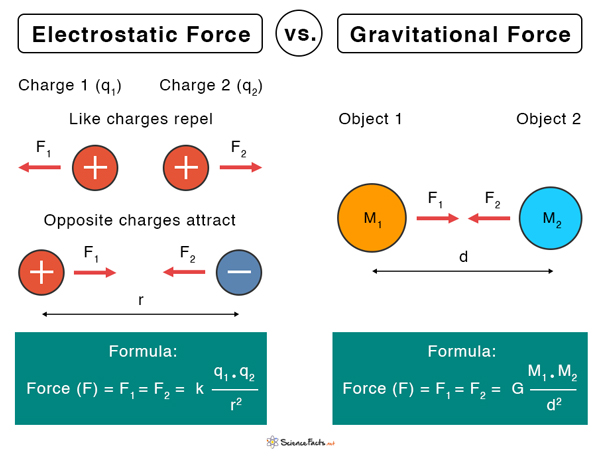
Difference between Electrostatic and Gravitational Forces
Electrostatic Force Problems and Solutions
Directly proportional to the product of the magnitude of the chargesInversely proportional to the square of the distance between the two charges
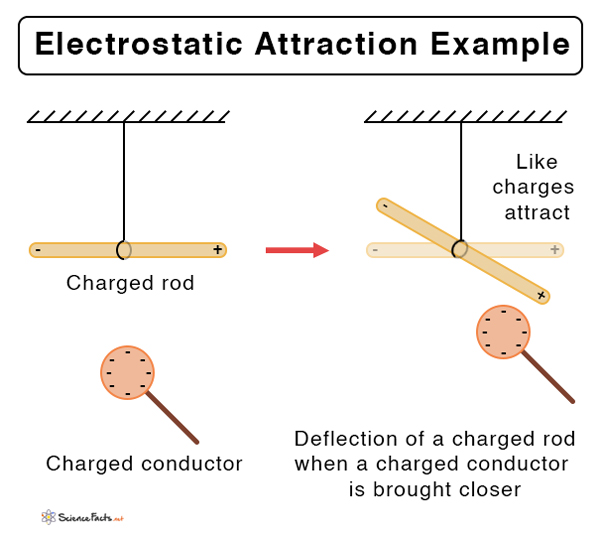
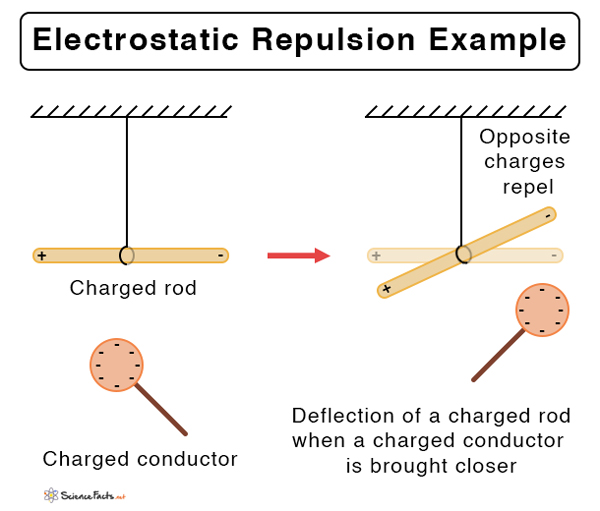
Suppose the two charged particles are brought close to one another. There will be an attraction if the charges are opposite, or if one is positive and the other negative. On the contrary, the charges will repel if both of them are positive or negative. In other words, like charges repel and unlike charges attract.
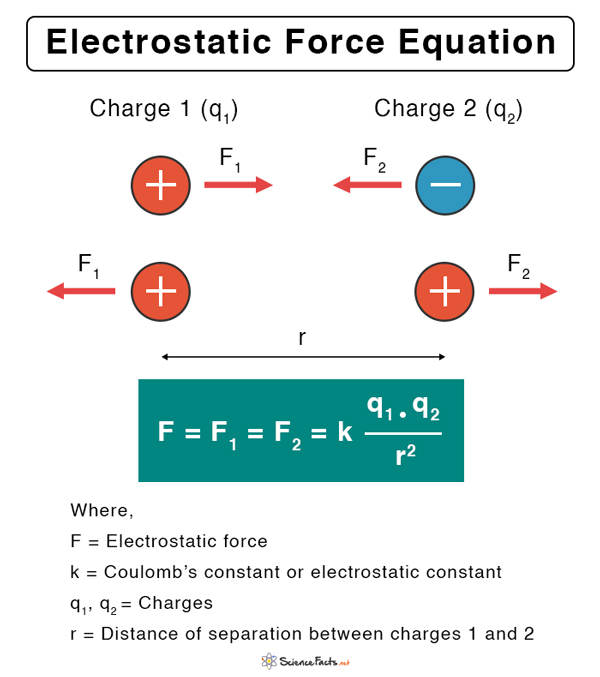
Electrostatic Force Equation
Let us assume that q1 and q2 are the amounts of charges on the two particles separated by a distance r. According to Coulomb’s law, the electrostatic force between the two charges is given by the following equation. Electrostatic force unit: N (Newton). Here, k is called Coulomb’s constant. Its value is 9 x 109 N.m2.C-2. Generally, q1 and q2 can be positive or negative. When two opposite point charges are placed close to each other, the force is attractive and hence, its sign is negative. The magnitude is merely the value of F without the sign. According to the above equation, F vanishes when r → ∞. Hence, at an infinitely large distance, the electrostatic force is zero. Technically, the range of F is infinite. The work done W by the force F on a particle is the product of the force and the displacement d. W= F x d The work done in displacing the particle from one position to another is independent of the path taken. Hence, the electrostatic force is conservative.
Like charges repel, and opposite charges attractDirectly proportional to the product of two point-chargesInversely proportional to the distance of separation between the chargesActs along the line joining the two charges
Rubbing of clouds generate charges. These charges will neutralize by passing through the atmosphere until they reach the neutral ground. We perceive this as lightning.After combing, if we bring the comb close to a piece of paper, there is a force of attraction between them.
A silk shirt clings to the body because of charged particles on the shirt. The same applies to a woolen sweater when taking off.Getting out of a car on a warm, dry day and touching the door give us charges.Grains of sugar are attracted to the inside surface of a container due to electrostatic forces.
PhotocopierLaser and ink-jet printersVan der Graaff generatorSmoke precipitator andCCD camera
Solution. The ratio of electrostatic force to the gravitational force of two electrons is given by FE/FG = ke2/Gme2 = 9 x 109 N.m2.C-2x (1.6 x 10-19)2 C2/6.67 x 10-11 N.m2.kg-2x (9.1 x 10-31)2 kg2 = 4 x 1042 Therefore, the relationship between the gravitational force and the electrostatic force is FE = 4 x 1042 FG Problem 2. If the electrostatic force between two charges is 124 N. What is the distance of separation between the charges, if the charges are 4 μC and 9 μC? Given k = 9 x 109 N m2.C-2. Solution. We shall use the following equation. F = k q1 q2/r2 Given, q1 = 4 x 10-6 C, q2 = 9 x 10-6 C, F = 124 N and k = 9 x 109 N m2.C-2. Putting these values, 124 N =9 x 109 N m2.C-2 x 9 x 10-6 C x 4 x 10-6 C / r2 Or, r2 = 9 x 109 N m2.C-2 x 9 x 10-6 C x 4 x 10-6 C / 124 N = 0.00261 m2 Or, r = 0.05 m