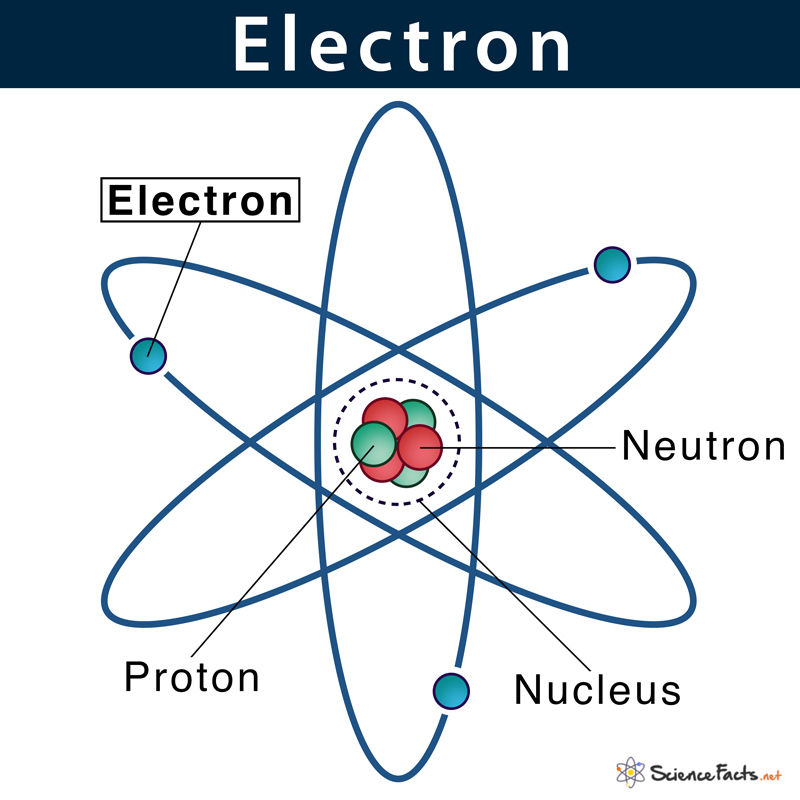
Given below is the structure of an atom, where electrons are moving around the nucleus. This pattern is similar to the way planets in the solar system revolve around the sun.
Who Discovered Electron
Where are they Found in an Atom
Characteristics
- Charge: They are negatively charged with a charge of -1, which is equal and opposite to the charge of a proton.
- Mass: Has a mass of 9.1094 x 10-28 g, which is almost 1/2000 times the mass of proton or neutron. Thus contribute very less to the total mass of an atom.
- Number in Atoms: All atoms have the same number of electrons as protons, thus helping the cell become electrically neutral. Thus in a electrically neutral atom, Number of electrons (e–) = Number of protons (p+) For example, the nucleus of Osmium contains 76 electrons.
- Movement: The force of attraction between the oppositely charged electrons and protons helps the former move in the space around the nucleus in their specified orbits.