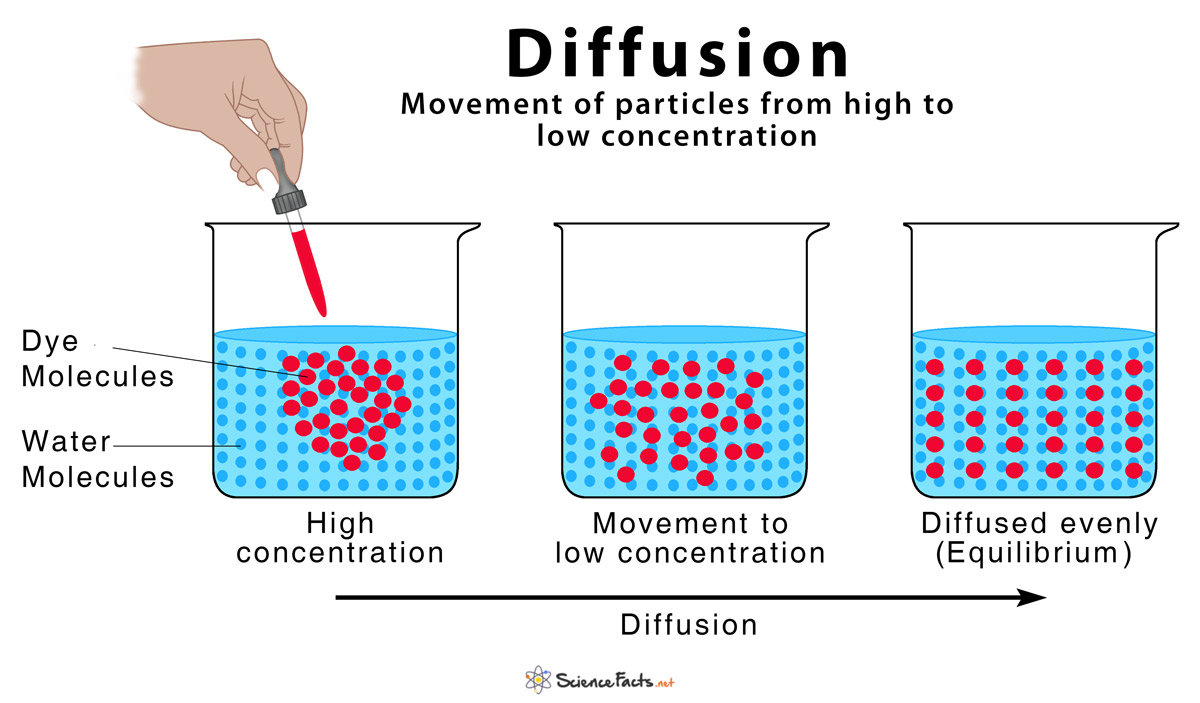
What Causes Diffusion and What Happens During the Process
Basic Characteristics of Diffusion
What Factors Affect Diffusion
What are the Different Types of Diffusion
If Fick’s laws can describe a diffusion process, it is called a normal or Fickian diffusion, otherwise, it is named as anomalous or non-Fickian diffusion.
Examples of Diffusion
b) Respiration – The balance between oxygen and carbon dioxide within the cell is maintained by removing the excess carbon dioxide from the blood c) Excretion – Waste products are eliminated from the body d) Cellular Transport – Essential ions, small molecules, food, water, and minerals are taken up inside the cell
- Temperature: Warmer the temperature, higher is the rate of diffusion.
- Area of Interaction: More the surface area of interacting molecules, higher is the rate of diffusion.
- The Extent of the Concentration Gradient: Greater the difference in concentration between the regions, higher is the rate of diffusion.
- Diffusion Distance: Smaller the distance covered by the diffusing molecules, faster is the rate of diffusion.
- Types of Diffusing Materials: At a particular temperature, materials with lighter atoms diffuse faster than heavier ones.
- Particle Size: At any given temperature, the diffusion of a smaller particle will be more rapid than the larger ones.
- Simple Diffusion It is the process in which substances move across a biologically active semi-permeable membrane along the concentration gradient without the involvement of any other molecules. Example: Breathing in oxygen and releasing carbon dioxide out of the body during respiration
- Facilitated Diffusion It is the process in which the diffusing material requires the presence of another molecule or a facilitator to perform diffusion. Example: Glucose, sodium ions, and potassium ions are transported in and out of the cell with the help of specific carrier proteins and protein channels