Examples
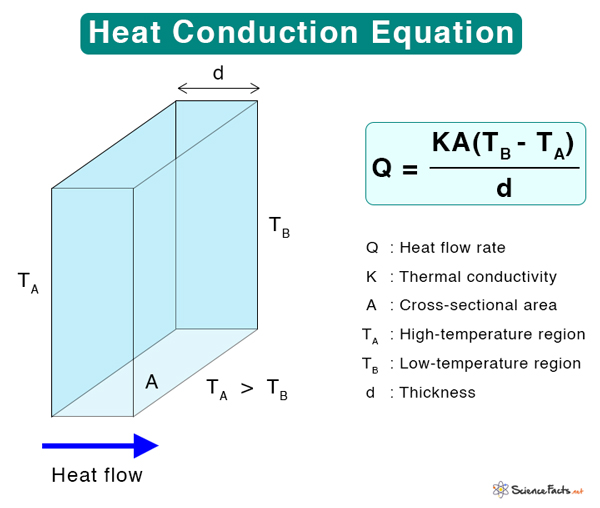
Equation
Thermal Conductivity
According to kinetic theory, matter is made of particles that are in constant random motion. This motion manifests as thermal energy, which depends on the temperature. The higher the temperature, the higher the thermal energy. The motion of particles, whether translatory or vibrational, leads to collisions. The particles transfer energy among themselves. Consequently, heat travels from a high-temperature region to a low-temperature region. Where Q: Heat transfer rate (Js-1 or W) K: Thermal conductivity (Wm-1K-1) A: Cross-sectional area (m2) ( \frac{\Delta T}{\Delta x} ): Temperature gradient (Km-1) Suppose heat flows through a conductor of thickness d whose ends are at temperatures TA (hot) and TB (cold). The heat flowing per second through the conductor is K = Q Thus, thermal conductivity is defined as the amount of heat flowing through a conductor of unit length, whose cross-section has a unit area and whose ends are at a unit temperature difference. Metals have high thermal conductivity because their valence electrons are delocalized and can efficiently conduct heat. For example, the thermal conductivities of silver and copper are 406 Wm-1K-1 and 385 Wm-1K-1, respectively. Insulators are poor conductors of heat. They have voids in between the atoms, which interfere with heat transfer. For example, the thermal conductivity of wood ranges from 0.04 to 0.12 Wm-1K-1. Air is a poor conductor of heat. Its thermal conductivity at 0 ˚C is 0.024 Wm-1K-1.