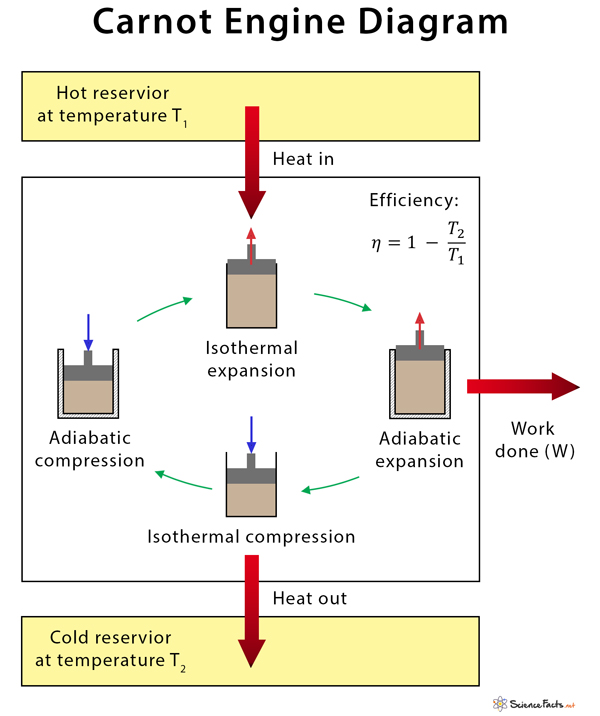
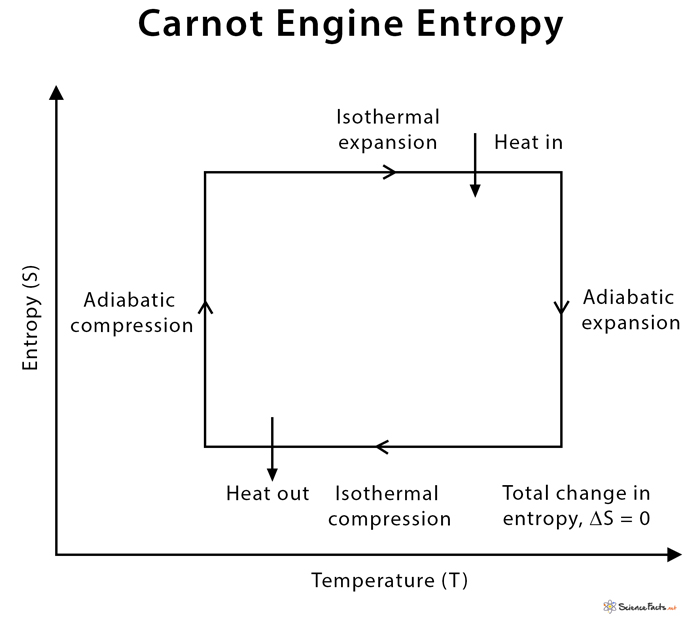
The Carnot engine was devised by French engineer and scientist Nicolas Léonard Sadi Carnot in 1824. During the working of the Carnot engine, the system is taken from temperature T1 to T2 and then back from temperature T2 to T1. It is best represented by a pressure-volume (PV) diagram.
Carnot Refrigerator
The thermodynamic cycle that is observed in the Carnot engine is a reversible process. The engine can be acted upon by an external force to transfer heat. Thus, the Carnot engine works like a refrigerator. Heat is sucked out of the cold reservoir inside a fridge. Electrical energy (work input) is supplied from the wall outlet. There is some waste heat dumped into the kitchen (surrounding). Qc is the heat that is wasted and dumped into the cold reservoir. The following formula gives the efficiency of a Carnot engine. The conceptual thinking of the Carnot cycle is that it establishes the maximum possible efficiency for any heat engine cycle operating between T1 and T2. The reason is that there is no friction between the piston and the walls of the cylinder. It runs on reversible processes, which yields maximum work output for a given heat input. However, the Carnot engine cannot achieve 100% thermal efficiency because some waste heat is always produced during its operation.
To evaluate real engines in the context of their theoretical maximum efficiency, especially during the design.As a theoretical reference cycle to compare the efficiencies of the actual Otto (petrol) and Diesel cycles.To convert oceanic thermal energy into work.
However, the Carnot engine is impractical because the heat transfer into the engine in the isothermal process is too slow to be of practical value.