Theory
Equation
Types of Atomic Spectroscopy
Applications
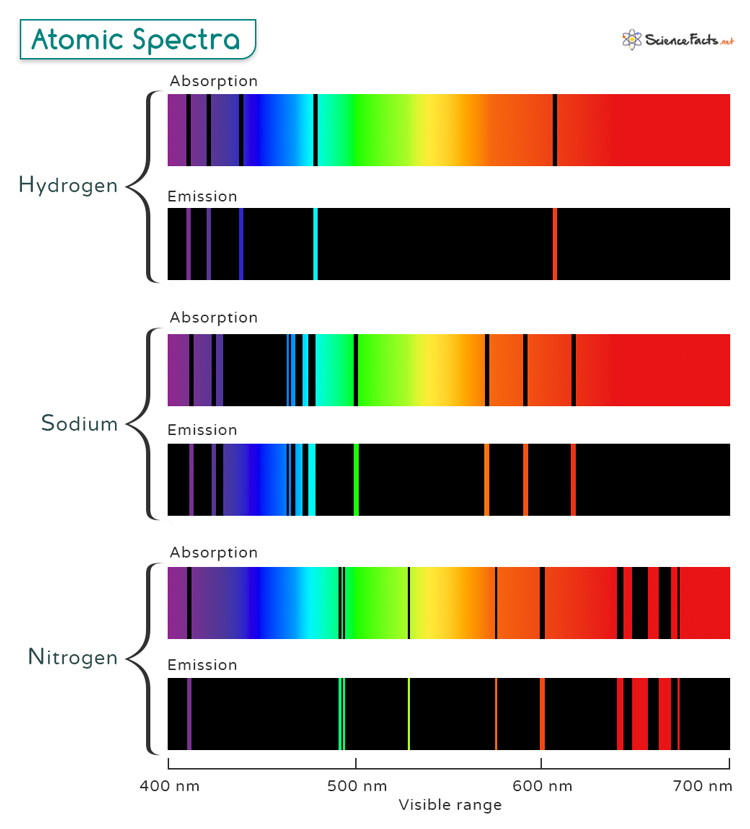
The Bohr model of the atom describes electrons orbiting the nucleus in circular paths, each with a unique energy level. When an electron transitions from a higher energy level to a lower one, it releases a photon with an energy equal to the difference between the two levels. This photon has a specific wavelength determined by the formula Where h is Planck’s constant, c is the speed of light, and λ is the wavelength. Therefore, atomic spectra are a direct consequence of the quantized nature of an atom’s energy levels, as described by quantum mechanics. Atomic spectra exhibit distinct, discrete lines rather than a continuous range of wavelengths. This specific pattern of wavelengths is known as its line spectrum, which is unique to each element. This line spectrum contrasts with the continuous spectrum of light emitted by hot, dense objects like the sun. Analyzing these spectral lines provides valuable information about the element’s structure and composition. The technique used for such analysis is called atomic spectroscopy. The Rydberg Formula is expressed as: Where:
λ is the wavelength of the emitted lightRH is the Rydberg constant, a physical constant with a value of approximately 1.097 x 10⁷ m⁻¹n₁ and n₂ are the initial and final energy levels of the electron, respectively
This equation accurately predicts the wavelengths of the spectral series observed in the hydrogen emission spectrum, including the Lyman, Balmer, Paschen, and Brackett series. AES can be used for both qualitative and quantitative analysis. It is commonly used in environmental monitoring, materials science, and food and pharmaceutical analysis. One key advantage of AES is its ability to detect and measure trace elements, making it a valuable tool for various industries. Additionally, AES is a relatively simple and cost-effective technique.
2. Atomic Absorption Spectroscopy
Atomic absorption spectroscopy (AAS) is a widely used analytical technique for quantifying trace elements in various samples. It relies on the principle of light absorption by free atoms in the gaseous state. AAS involves atomizing the sample through a flame or furnace to convert the analyte elements into free, unexcited atoms in the ground state. These atoms then absorb light at specific wavelengths, with the amount of absorption being proportional to the element’s concentration, as per the Beer-Lambert law. AAS is highly sensitive and selective, enabling the analysis of trace elements at parts-per-million or parts-per-billion levels. It is widely used in environmental monitoring, food and beverage analysis, clinical diagnostics, and materials science.