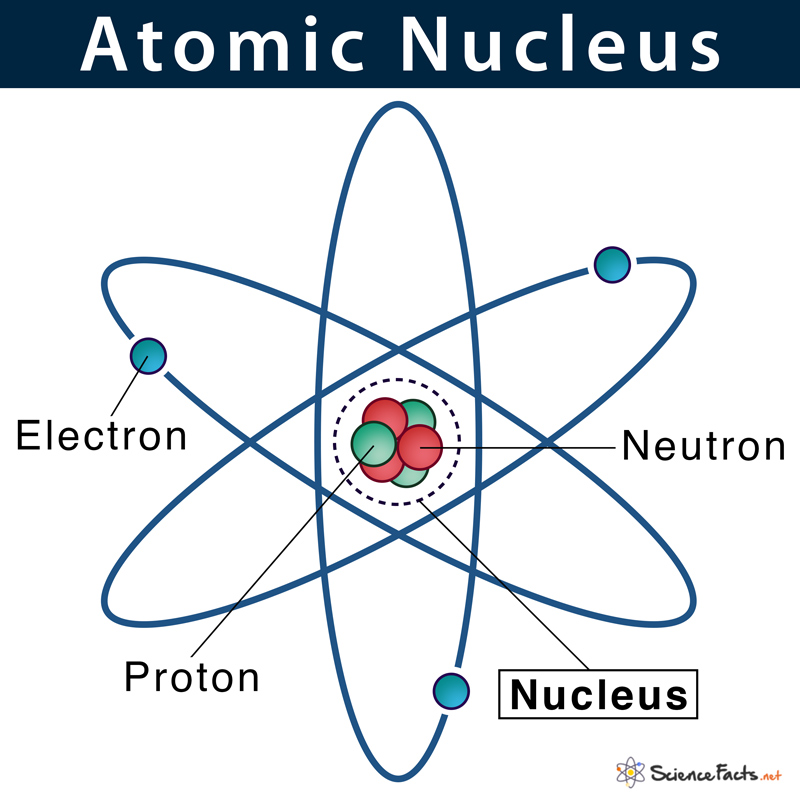
The word ‘nucleus’ means ‘kernel of a nut’. In 1844, Michael Faraday used the nucleus to describe the ‘central point of an atom’. Ernest Rutherford described the exact meaning of atoms in 1912.
Who Discovered the Nucleus?
Its Size
What are Nucleus Made of
Charge of the Nucleus
Mass of Nucleus
What Makes Nucleus Stable
Regular units of mass, such as gram (g) or Kilogram (Kg), cannot weigh anything as small as an atom. Thus, scientists have created a new unit of mass. It is called the Atomic Mass Unit (u). Carbon-12 is taken as its reference, and one amu is equal to 1/12th the weight of one atom of Carbon 12. 1 amu = 1.660539 10-24 g